The U.S. Food and Drug Administration (FDA) recently published its guidelines related to citizen petitions and petitions for stays of action under section 505(q) of the federal Food, Drug, and Cosmetic Act (a "Petition"). These guidelines detail how the FDA determines if the provisions of section 505(q) apply to a Petition and whether the certification and the verification required for filing with any Petition or supplemental information or comments meet statutory requirements. The FDA also commented on the relationship between review of such Petitions and review of abbreviated new drug application (ANDA) and section 505(b)(2) applications for which the agency has not made a final approvability determination.
Does Section 505(q) Apply to the Petition?
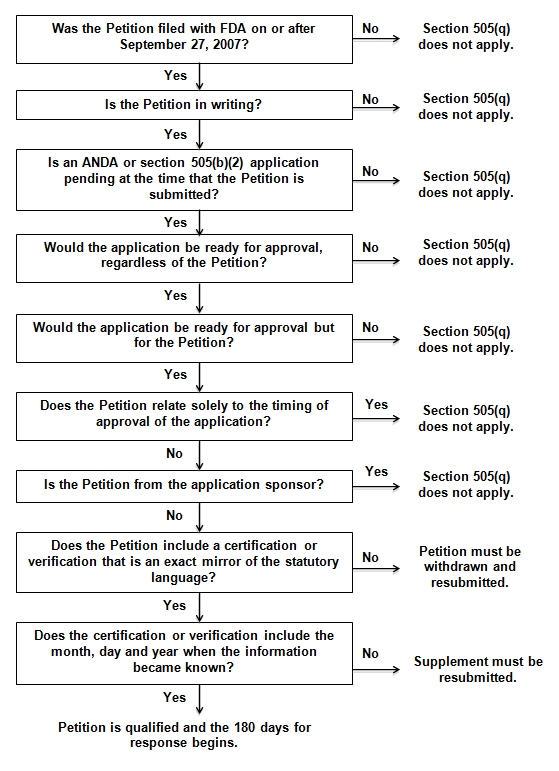
The FDA has determined that section 505(q) applies to Petitions only when the Petition was submitted to the FDA on or after September 27, 2007; the Petition is submitted in writing; an ANDA or section 505(b)(2) application is pending at the time the Petition is submitted; the petitioner requests an action that could delay approval of such application; and the Petition does not fall within an exception to section 505(q).
The FDA considers a Petition "in writing" when it complies with section 10.30 of FDA regulations related to citizen petitions or section 10.35 of FDA regulations related to requests for administrative stay of action. Moreover, the FDA will not permit any cross-references or reliance upon information outside of the Petition. In other words, the Petition has to be able to stand alone and contain all information relating to the Petition, both favorable and unfavorable.
For a Petition to be considered an "action that could delay approval of a pending ANDA or 505(b)(2) application" the FDA considers the following but for test:
- Would the application be ready for approval regardless of the Petition? If not, section 505(q) does not apply; and
- Would the application be ready for approval but for the Petition? If so, section 505(q) applies.
The FDA will then review whether the delay is necessary to protect the public health. The FDA considers the following in making such a determination:
If the application were approved before the Agency completed the substantive review of the issues in the petition and, after further review, the Agency concluded that the petitioner's arguments against approval were meritorious, could the presence on the market of drug products that did not meet the requirements for approval negatively affect the public health?
If the FDA determines that the but for test places the Petition within section 505(q), the FDA will notify the applicant within 30 days that such a determination has been made and, if applicable, of any clarification or additional data needed from the applicant to allow the FDA to review the Petition promptly. The FDA will also provide a brief summary of the substantive issues raised in the Petition. However, the FDA does not intend to notify a petitioner of any public health delays because section 505(q) does not require such a notification.
Finally, section 505(q) will not apply to Petitions that "relate solely to the timing of approval of an application pursuant to the 180-day exclusivity provision. . . of the Act, or are from the sponsor of [an] application and seek only to have the FDA take or refrain from taking any action with respect to that application."
Does the Certification or Verification Meet the Statutory Requirements?
The FDA will accept a Petition or supplemental information and comments only where the certification or verification "exactly mirrors the language provided in section 505(q)." Additionally, the FDA requires that the petitioner provide the month, day and year "on which the information first became known to the party on whose behalf the petition is submitted." A month and year alone will not suffice and will be found deficient. However, "[t]o the extent that a petitioner believes further explanation of the date is needed, [the FDA] believe[s] that the blank space in the certification allows for the insertion of additional information."
If the certification is deficient or is not included with the original Petition, the petitioner should "(1) submit a letter withdrawing the deficient petition pursuant to § 10.20(g) and (2) submit a new petition that contains the certification." Merely filing a supplement to add the certification will not cure this deficiency. The 180-day period for response to a Petition will "begin from the date of submission of the new, complete petition and not the original, deficient petition." Likewise, when a verification is deficient, the petitioner is required to resubmit the supplemental information or comments with the required verification. The petitioner cannot rely on the FDA to notify him or her of a deficient verification. The FDA places the responsibility on petitioners to ensure their submissions comply with the statute.
What Is the Relationship Between Review of a Petition and Review of an Application Without a Final Approvability Determination?
Section 505(q) requires that the FDA takes final action on a Petition within 180 days of its submission. However, review of the application itself falls under a separate review process that may not be completed by the date a response to the Petition is due. The FDA "do[es] not interpret section 505(q) to require a substantive final Agency decision within 180 days on the approvability of a specific aspect of a pending application when a final decision on the approvability of the application as a whole has not yet been made." In that situation, the FDA would likely "deny a petition without comment on the substantive approval issue."
For Further Information
If you have any questions about this Alert, please contact Frederick (Rick) R. Ball, Carolyn A. Alenci, any member of the Pharmaceutical, Pharmacy & Food industry group or the attorney in the firm with whom you are regularly in contact.
Disclaimer: This Alert has been prepared and published for informational purposes only and is not offered, nor should be construed, as legal advice. For more information, please see the firm's full disclaimer.